PVCHR intervention at NHRC on FOPL and NCDs (case no. 4227/90/0/2021)
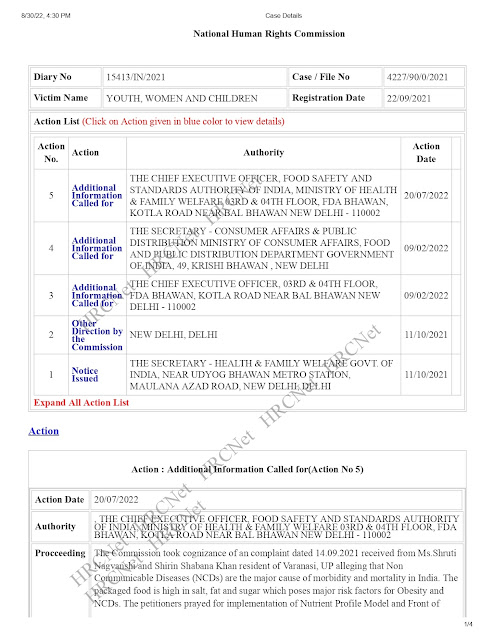
The Commission took cognizance of an complaint dated 14.09.2021 received from Ms.Shruti Nagvanshi and Shirin Shabana Khan resident of Varanasi, UP alleging that Non Communicable Diseases (NCDs) are the major cause of morbidity and mortality in India. The packaged food is high in salt, fat and sugar which poses major risk factors for Obesity and NCDs. The petitioners prayed for implementation of Nutrient Profile Model and Front of Packet Labelling (FOPL) regulation in India as it will help the food processing industry towards healthy food.
Pursuant to the directions of the Commission, Dy. Director Legal Metrology, Weights and Measures Unit, Deptt. of Consumer Affairs, Min. of Consumer Affairs, Food & Public Distribution vide letter dated 08.04.2022 submitted that as per Legal Metrology (Packaged Commodities) Rules 2011, Rule 6 states that: The Commission took cognizance of an complaint dated 14.09.2021 received from Ms. Shruti Nagvanshi and Shirin Shabana Khan resident of Varanasi, UP alleging that Non Communicable Diseases (NCDs) are the major cause of morbidity and mortality in India. The packaged food is high in salt, fat and sugar which poses major risk factors for Obesity and NCDs. The petitioners prayed for implementation of Nutrient Profile Model and Front of Packet Labelling (FOPL) regulation in India as it will help the food processing industry towards healthy food.
(1) Every package shall bear thereon or on label securely affixed thereto, a definite plain and conspicuous declaration made in accordance with the provisions of this chapter as, to
Pursuant to the directions of the Commission, Dy. Director Legal Metrology, Weights and Measures Unit, Deptt. of Consumer Affairs, Min. of Consumer Affairs, Food & Public Distribution vide letter dated 08.04.2022 submitted that as per Legal Metrology (Packaged Commodities) Rules 2011, Rule 6 states that:
a) The name and address of the manufacturer and packer and for any imported package the name and address of the importer.
(1) Every package shall bear thereon or on label securely affixed thereto, a definite plain and conspicuous declaration made in accordance with the provisions of this chapter as, to
b) The common or generic names of the commodity contained in the package. The name and number of quantity of each product.
a) The name and address of the manufacturer and packer and for any imported package the name and address of the importer.
c) The net quantity, in terms of the standard unit of weight or measure. The number of the commodity contained in the package shall be mentioned.
b) The common or generic names of the commodity contained in the package. The name and number of quantity of each product.
d) The month and year in which commodity is manufactured or pre- packed or imported shall be mentioned in the package:
c) The net quantity, in terms of the standard unit of weight or measure. The number of the commodity contained in the package shall be mentioned.
(i) Provided that the provisions of the Prevention of Food Adulteration Act 1954 (37 of 1954) and the rule made there under shall apply.
d) The month and year in which commodity is manufactured or pre- packed or imported shall be mentioned in the package:
(i) Provided that the provisions of the Prevention of Food Adulteration Act 1954 (37 of 1954) and the rule made there under shall apply.
(ii) Provided further that nothing in this sub-clause shall apply in case of packages containing seeds.
(ii) Provided further that nothing in this sub-clause shall apply in case of packages containing seeds.
(iii) Provided that a manufacturer may indicate the month and year using a rubber stamp without overwriting.
(iii) Provided that a manufacturer may indicate the month and year using a rubber stamp without overwriting.
(iv) Provided that for packages containing cosmetics products the provisions of the Drugs and Cosmetics Rules,1945 shall apply.
(e) If a package contains a commodity which may become unfit for human consumption after a period time, the best before or use by date, month and year shall also be mentioned on the label. provided that nothing in this clause shall apply if a provisions in this regard is made in any other law.
(iv) Provided that for packages containing cosmetics products the provisions of the Drugs and Cosmetics Rules,1945 shall apply.
Assistant Director(Legal), Food Safety and Standards Authority of India, Ministry of Health & Family Welfare, New Delhi vide detailed report dated nil received in the Commission on 04.05.2022 has submitted as under:-
(e) If a package contains a commodity which may become unfit for human consumption after a period time, the best before or use by date, month and year shall also be mentioned on the label. provided that nothing in this clause shall apply if a provisions in this regard is made in any other law.
i) That the Food Safety and Standards Act, 2006 was promulgated to consolidate the laws relating to food and to regulate their manufacture, storage, distribution, sale and import etc.
Assistant Director(Legal), Food Safety and Standards Authority of India, Ministry of Health & Family Welfare, New Delhi vide detailed report dated nil received in the Commission on 04.05.2022 has submitted as under:-
i) That the Food Safety and Standards Act, 2006 was promulgated to consolidate the laws relating to food and to regulate their manufacture, storage, distribution, sale and import etc.
ii) The Regulation also mandate that every package of food should carry information viz. name of food, list of ingredients etc. used in the product in descending order of their composition etc.
ii) The Regulation also mandate that every package of food should carry information viz. name of food, list of ingredients etc. used in the product in descending order of their composition etc.
iii) Front Package Labels(FOPL) provides simple nutrition information and more accessible portion food package instead its back side.
Moreover, FSSAI through its Scientific Panels well Stakeholders’ Consultations deliberated on matter of Pack Labelling (FOPL), whereupon WHO-SEARO(South-East Asian Region trans and energy-SEARO Model. Stakeholders, certain industry associations also submitted comments and raised concern specified thresholds levels sugar. Meanwhile the Ministry of Food Processing Industries (MOFPI) has advised FSSAL to constitute working group review the thresholds.
iv) Food Authority constituted consultative group and consensus emerged on nutrients concern. Accordingly to specify threshold was referred to Scientific Panel. Furthermore, FSSAI awarded work to IIM-Ahemdabad to carry out consumer survey to analyse major FOPL models available across the globe and behavioral change in Indian Consumers on a National Level. The study report has been presented to the consultative group forum to provide comments. Regulations in this regard will be framed and processed in a consultative manner as provided for in the FSS Act, 2006. The most countries in the world have adopted mandatory display of nutritional information on the back of the pack only and very few have mandated front of the pack labeling so far.
Moreover, FSSAI through its Scientific Panels well Stakeholders’ Consultations deliberated on matter of Pack Labelling (FOPL), whereupon WHO-SEARO(South-East Asian Region trans and energy-SEARO Model. Stakeholders, certain industry associations also submitted comments and raised concern specified thresholds levels sugar. Meanwhile the Ministry of Food Processing Industries (MOFPI) has advised FSSAL to constitute working group review the thresholds.
iv) Food Authority constituted consultative group and consensus emerged on nutrients concern. Accordingly to specify threshold was referred to Scientific Panel. Furthermore, FSSAI awarded work to IIM-Ahemdabad to carry out consumer survey to analyse major FOPL models available across the globe and behavioral change in Indian Consumers on a National Level. The study report has been presented to the consultative group forum to provide comments. Regulations in this regard will be framed and processed in a consultative manner as provided for in the FSS Act, 2006. The most countries in the world have adopted mandatory display of nutritional information on the back of the pack only and very few have mandated front of the pack labeling so far.
Commission has perused report. Let the outcome on the basis of Consultative Group Forum be intimated to the Commission within a period of eight weeks.
Commission has perused report. Let the outcome on the basis of Consultative Group Forum be intimated to the Commission within a period of eight weeks.
List after three months.






Comments
Post a Comment